E-SWIN IRPL Treatment E-EYE IRPL Treatment
E-Eye®/ IRPL® Patented Intense Regulated Pulsed Light technology provides patients with a treatment solution delivering long-lasting benefits.
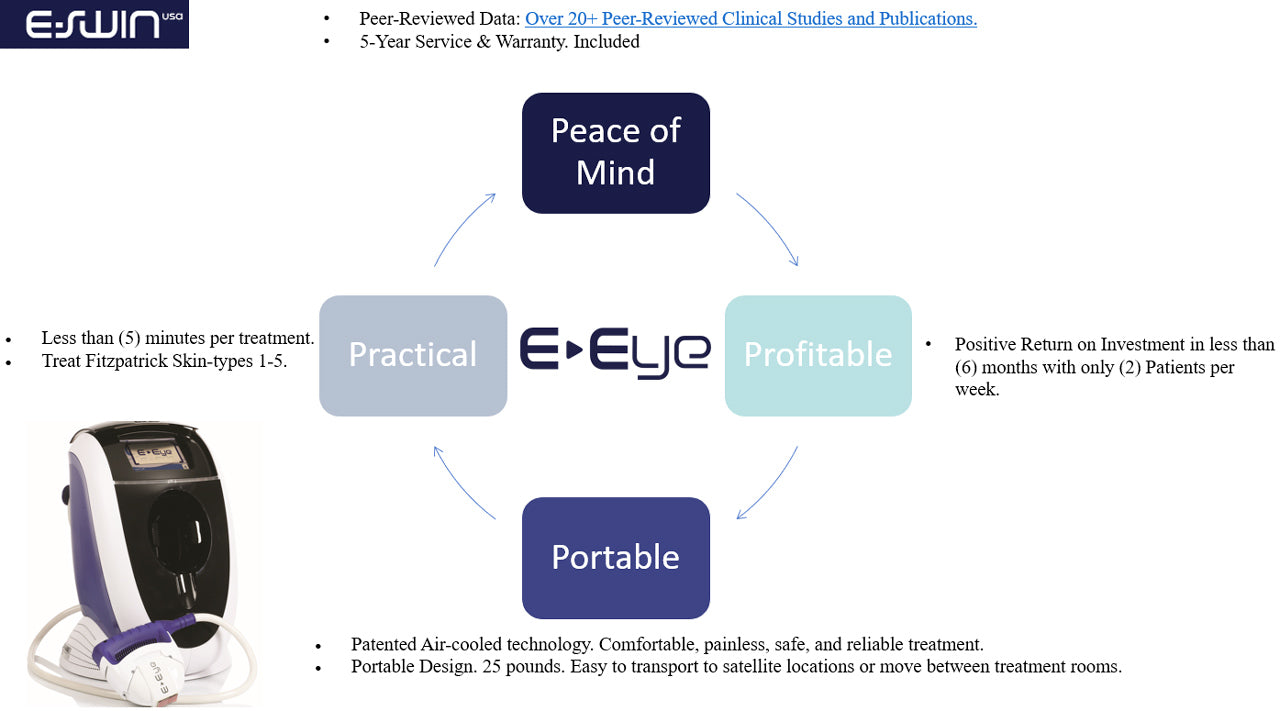
E-Eye is an FDA-cleared medical device under 510(k) (K200616) issued June 2020 with the indication for rosacea treatment. Designed and manufactured in France by E-SWIN, a leading manufacturer of high-tech medical equipment, non-invasive, entirely painless and completely harmless.
It generates Intense Regulated Pulsed Light by producing perfectly calibrated and uniformly sequenced light pulses. These sculpted pulses are delivered in the form of a train of pulses. The energy, spectrum, and time period are precisely adjusted to stimulate the Meibomian glands, allowing them to regain normal function.
Q453,783.91
Q272,270.35
The E-Eye is the first medically certified and registered IPL engineered and designed for the periorbital area to treat Ocular Surface Disease.
E-Eye IRPL generates Intense Regulated Pulsed Light by producing perfectly calibrated and uniformly sequenced light pulses. These sculpted pulses are delivered in the form of a train of pulses. The energy, spectrum, and time period are precisely adjusted to target the root causes – chronic inflammation. E-EYE contributes to treating periocular inflammatory conditions such as Rosacea and telangiectasia, which may lead to ocular surface conditions such as Dry Eye.
E-Eye IRPL generates polychromatic pulsed light that is precisely calibrated and homogenously sequenced to deliver a train of pulses in a single flash. The technology delivers a continuum of light that distributes the energy evenly to avoid excessive transfer of heat in any particular area, protecting the skin from any heat damage, within wavelength spectrum of 580 nm – 1200 nm.
Since 2015, there has been thousands of E-Eye IRPL units installed worldwide, with a high success rate, scientific proven efficacy and no adverse events reported.

The treatment is performed within a short, efficient protocol of 3-4 sessions. The effect of the treatment appears very rapidly after each session and is cumulative. Usually the results last a minimum of 6 months. In order to improve the achieved results and to avoid returning discomfort, it is recommended to repeat single maintenance sessions on demand, with usually one single session per year.
This treatment protocol consists of 3 sessions (and a fourth optional session) as follows:
Day 1 / Day 15 / Day 45 / Day 75 (optional)
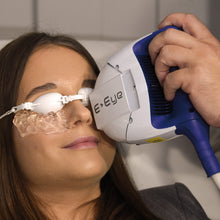
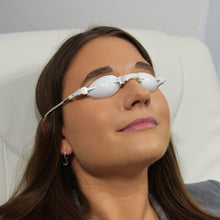
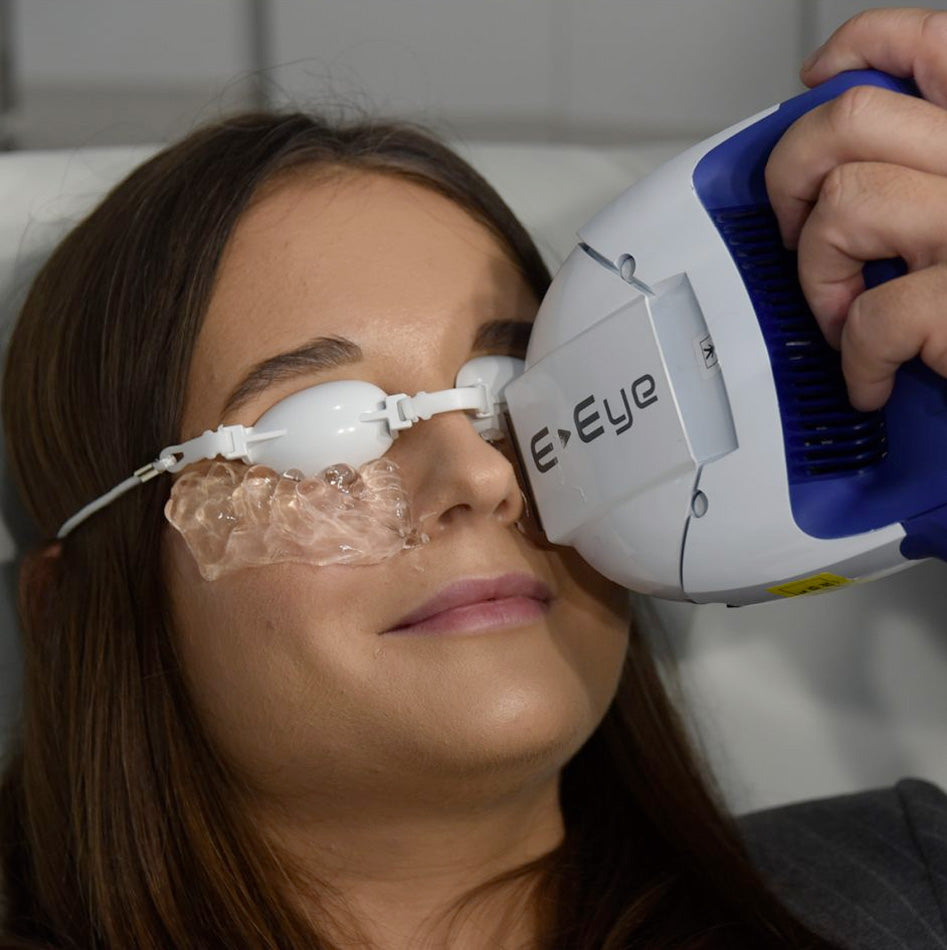
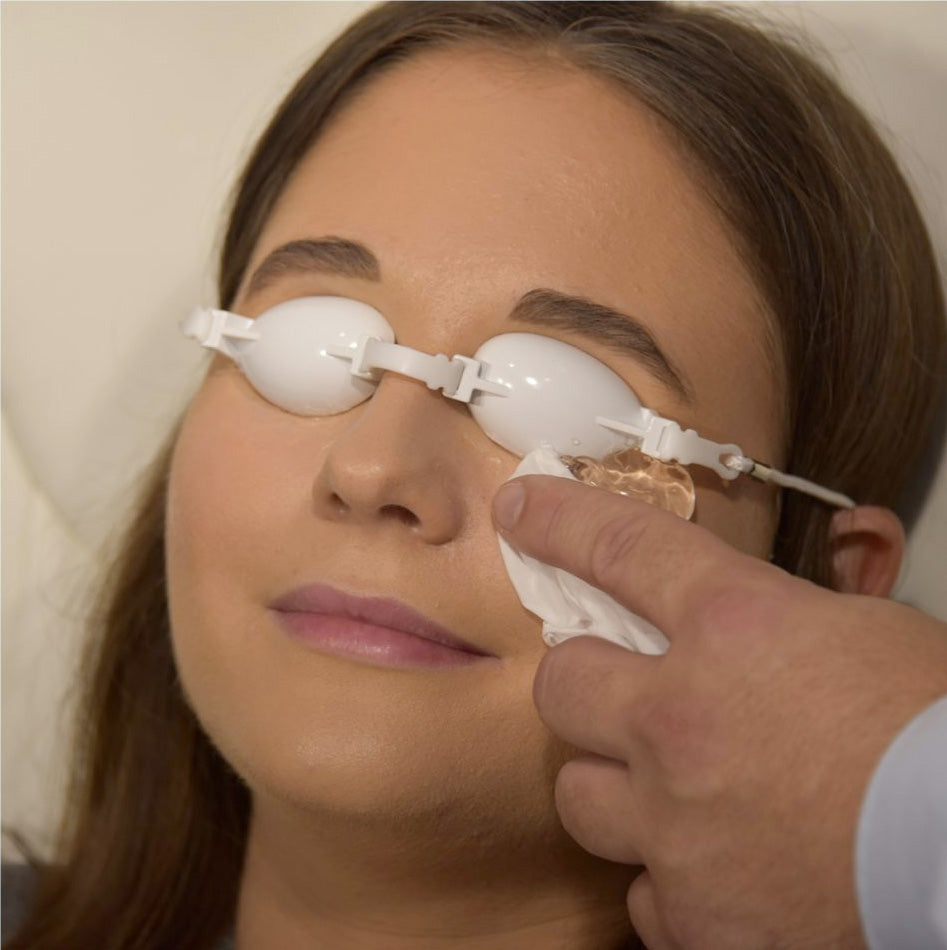
The periorbital treatment with E-Eye only takes a few minutes, with the patient comfortably seated in a chair. The patient's eyes are covered by protective eyecups, and hydrogel is applied to the lower eyelid. A series of gentle and non-invasive light pulses is then performed around the lower eye. This series is repeated in the same manner for both eyes.
The treatment aims to restore the normal activity of the Meibomian gland, resulting in rapid improvement for the patient after the very first session. The effects of the first two treatments typically last a few days up to 2 to 3 weeks. Long-lasting effects are expected to persist for a minimum of 6 months up to 3 years.
The E-Eye protocol typically comprises three to four sessions. It is recommended to repeat the application once symptoms begin to reappear.


As a result, the dry lipid layer receives a natural boost of lipids, which reduces the evaporation of tear fluid and prevents the eye from drying out. In addition, the quality of the glandular secretions is improved, and the tear film‘s lipid layer is stabilized.

E-Eye is a medical device specifically designed to treat dry eye syndrome caused by MGD (Meibomian Gland Dysfunction).
It generates Intense Regulated Pulsed Light by producing perfectly calibrated and evenly sequenced light pulses. These sculpted pulses are delivered in the form of a train of pulses. The energy, spectrum, and time period are precisely adjusted to stimulate the Meibomian glands, encouraging them to return to their normal function. E-Eye serves as a medical solution for treating and preventing dry eyes.
IPL technology has numerous advantages over laser treatment. IPL achieves equivalent clinical results with the reduced deployment of energy. Accordingly, the safety of the patient is considerably enhanced.
Conversely, however, there have been few overall advances in IPL technology over the past 15 years.
One of the major shortcomings is the absence of regularity and uniformity of the light emitted in flash treatment. The result is a lack of control in the thermal impact upon the target area.
The results achieved, although correct in the majority of cases, are not ideal.
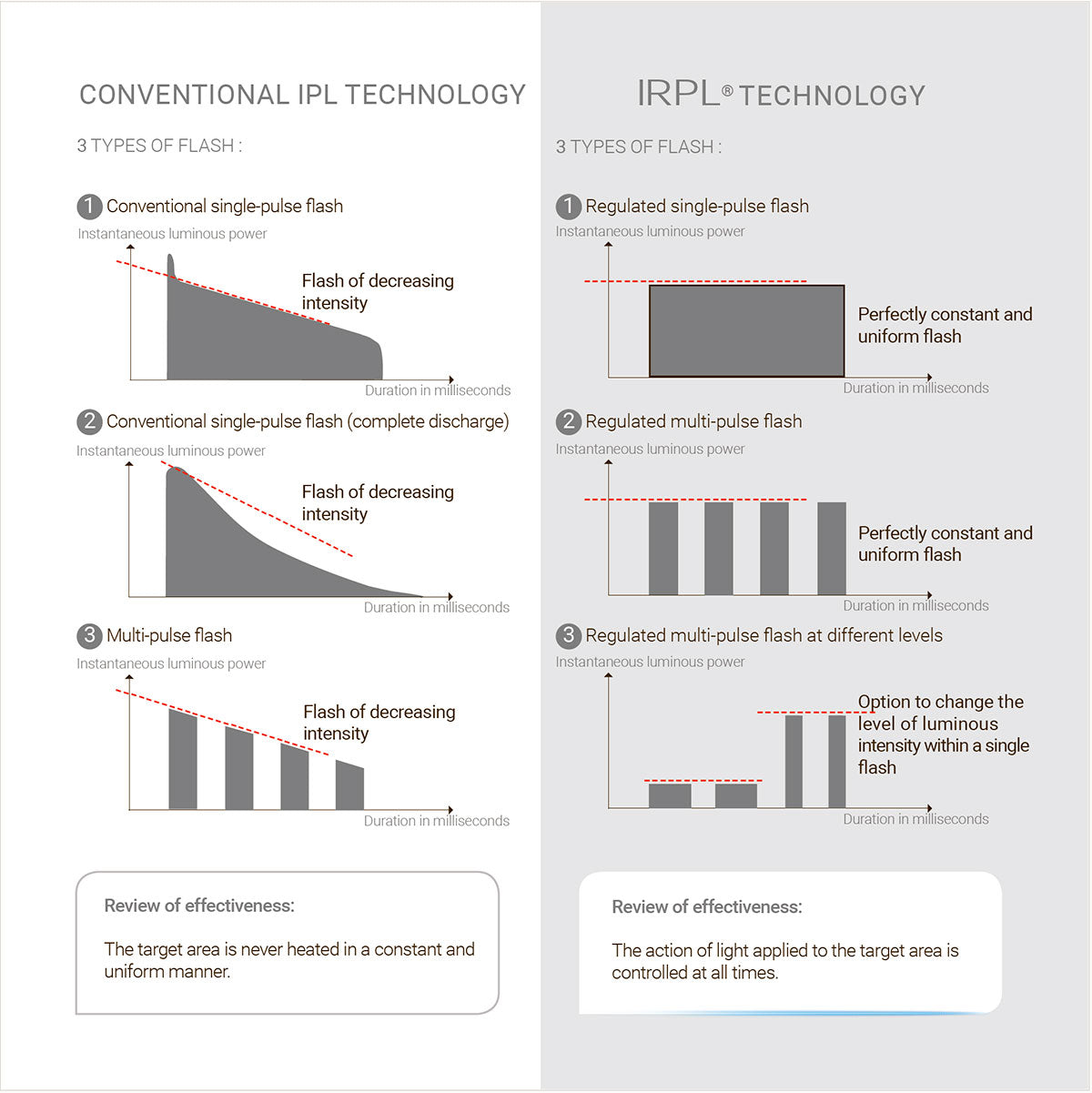
IRPL® technology developed by E-Swin engineers introduces an entirely new dimension to conventional IPL: « regulation ».
Regulated flash technology permits the generation of flashes, the instantaneous luminous power output of which remains constant throughout the duration of the flash concerned.
The uniformity permits the complete control of heat generated in the target area. Accordingly, this control further enhances the safety of flashes emitted.
It should be noted that IRPL® can go further still in the fine control of flash configuration. It is possible, within a single flash, to generate sub-flashes of different intensity. This offers unprecedented therapeutic possibilities, which are inconceivable with conventional IPL.
In brief, IRPL® represents the ultimate in terms of the control of light emitted by a flash lamp.
New therapeutic possibilities will be available as a result, specifically in the following fields: dry-eye syndrome, pigmentation disorders, acne, vascular disorders.
These will supplement everyday applications for permanent depilation and collagen stimulation.
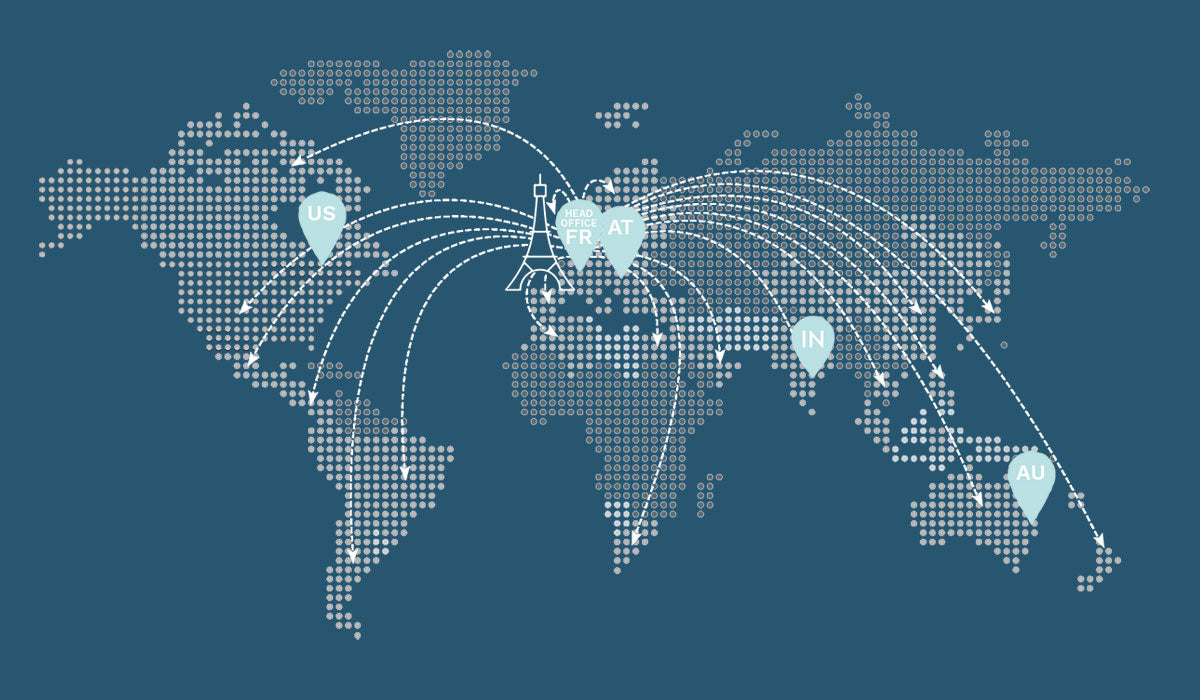
Since the start of international distribution in 2016, E-Eye has set up in many countries. E-Eye is a proven solution now available in more than 50 countries around the world!
In 2018, over 100,000 patients have been treated with the E-Eye, showcasing its widespread adoption and trust among users. Its efficacy spans across various severity levels of dry eye, from mild to severe, as validated by several clinical studies yielding highly significant results. Patients can expect long-term relief, typically lasting a minimum of 6 months, ensuring sustained comfort and improved eye health.
E-Eye was designed and developed to treat dry eye in a very efficient way, based on the innovative IRPL® (Intense Regulated Pulsed Light) technology, patent by E-Swin.
It only takes a few minutes to perform an E-Eye IRPL® treatment. The device is ready for use within seconds: it doesn’t require any pre-heating. Self-explaining settings on the touchscreen.
For optimal outcomes, it is recommended to conduct treatments on Day 1, Day 15, and Day 45. An optional treatment on Day 75 may be considered for cases of severe dry eye, ensuring comprehensive care and addressing varying patient needs effectively.
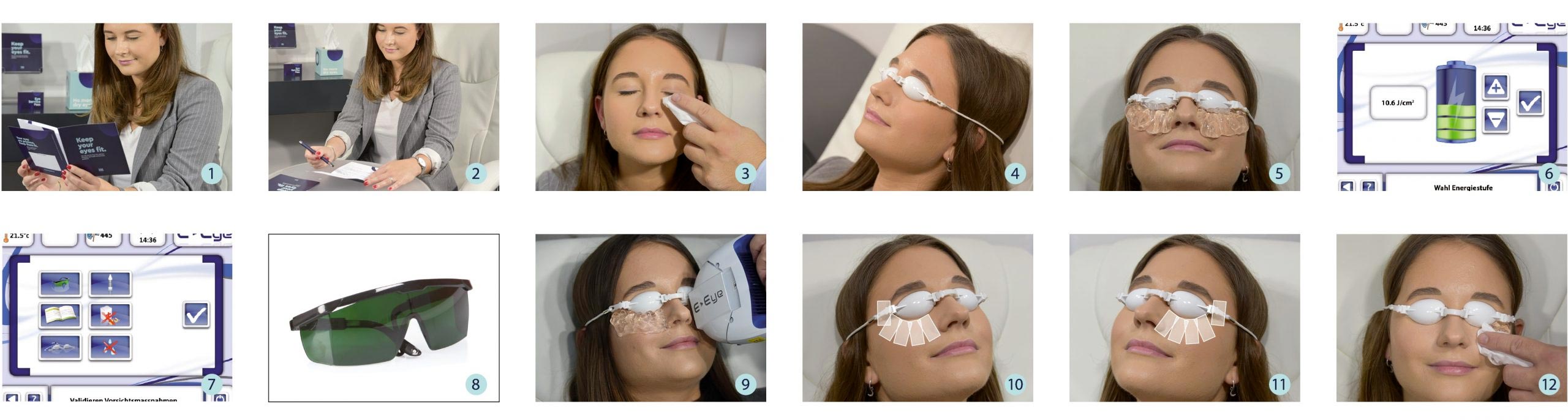
After a consultation with the patient, the practitioner will assess skin phototype, which is graded from I (very fair skin) to V (dark skin). A consent form is given to the patient before each treatment. It aims to inform and check for any contraindication.
TREATMENT
The patient is sitting comfortably on a treatment chair, preferably tilted.
After cleaning the skin from any make up, the eyes of the patient will be covered by protective goggles and the practitioner applies conductive gel on the cheekbone and the temporal areas. A series of 5 flashes is applied under the lower eyelid – starting from inner to outer side of the eye. The application is done the same on both eyes.
POST-TREATMENT
The patient is sitting comfortably on a treatment chair, preferably tilted.
E-Eye treatments are non-invasive, simple and safe. Patients generally experience a relief within a few minutes following the session. Make up can be applied again on the skin directly after the treatment. Patient can continue “daily life” right after the treatment.
E-EYE IRPL Treatment
Introduction ESW vision Dry Eye Management by Dr Liliana NÓBREGA
E-EYE IRPL Treatment
ESW vision The Future of Dry Eyes
E-EYE IRPL Treatment
Trainings video teaser

Click here to see the brochure

Click here to see the brochure

Click here to see the brochure

Click here to see the brochure

Click here to see the brochure
| Dimensions (length x width x height) | Max 29 x 18 x 24 in / Max 345 x 320 x 440 mm |
| Weight | Max 24 pounds / Max 11 kg |
| Noise Level | Max 55 dBA |
| Voltage | 100 – 240 VAC |
| Maximum Power Consumption | 540 VA |
| Frequency | 50/60 Hz |
| Usage temperature / Storage temperature |
+5°C – +25°C / -5°C – +65°C 41°F to 77°F / 23°F to 149°F |
| Authorized Humidity (without condensation) | 30 – 93 % |
| Dimensions (length x width x height) | Max 29 x 18 x 24 in / Max 345 x 320 x 440 mm |
| Weight | Max 24 pounds / Max 11 kg |
| Noise Level | Max 55 dBA |
| Voltage | 100 – 240 VAC |
| Maximum Power Consumption | 540 VA |
| Frequency | 50/60 Hz |
| Usage temperature / Storage temperature |
+5°C – +25°C / -5°C – +65°C 41°F to 77°F / 23°F to 149°F |
| Authorized Humidity (without condensation) | 30 – 93 % |
Introduction ESW vision Dry Eye Management by Dr Liliana NÓBREGA
ESW vision The Future of Dry Eyes
Trainings video teaser
KYLENE POLHAMUS, OD
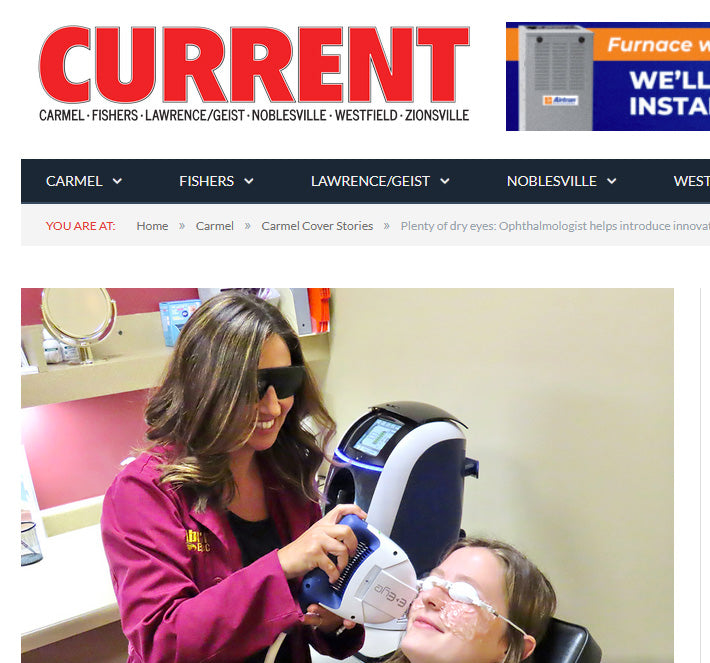
I am an optometrist with over 9 years of experience treating dry eye, and I can confidently say that the E-Eye / IRPL is by far my favorite treatment device I have ever used to treat dry eye due to meibomian gland dysfunction. It is a game charger for the majority of my patients and I love getting to see the positive effects it has on my patients, both objectively and subjectively, not-to-mention the case of use of the device and the excellent customer service that E-Swin provides.

Optometrist
Degrees:
B.S., Butler University, Indianapolis, IN
O.D., Indiana University School of Optometry, Bloomington, IN
Internships:
Eye Surgeons of Indiana, Indianapolis, IN
VA Northern Indiana Health Care System, Fort Wayne, IN
Indianapolis Eye Care Center, Indianapolis, IN
Atwater Eye Care Center, Bloomington, IN
Member:
American Optometric Association
Indiana Optometric Association
Optometry Divas
Dr. Kylene Polhamus is a graduate of Indiana University School of Optometry. Prior to optometry school, she received her B.S. degree from Butler University.
Dr. Polhamus is a certified member of the American Optometric Association, Indiana Optometric Association, and Optometry Divas. She is certified by the National Board of Examiners in Optometry and practices full scope optometry including the treatment and management of ocular disease, surgical co-management, and comprehensive medical and routine eye care for all ages. She has a special focus on dry eye syndrome.
She resides in Fishers and enjoys being active, traveling and spending time with her husband, 3 children and dog.
DR. DEE STEPHENSON, MD

DR. BRADLEY BARNETT